Is Nacl and Hcl a Buffer
Is HCl a buffer. I have a basic IHC question there is Tris-HCl buffer in the protocol I had gotten.

Which Of The Following Will Not Function As A Buffer Solution I Nacl And Naoh Ii Naoh And Nh4oh Iii Ch3coonh4 And Hcl Iv Borax And Boric Acid
The point is that HCl does not stick around and it gives ammonium chloride quantitatively.

. Today I make an experiment and realized that when I prepare 1M NaCl solution in 25mM Sodium Phosphate buffer pH75 NaCl lowered change the pH from 75 to 68. Yes it makes a bufferWeak base and its conjugated salt makes a buffer What is the balanced equation for the reaction of ammonium hydroxide with hydrochloric acid. This mixture assuming in water forms what is called a buffer solution.
HCHO 2 and NaCHO 2. Given is a solution of 1M NaCl and 1M HCl. NH 3 and NaOH.
For buffer solution rmpH rmpKa log left fracrmsaltrmacid right rmpKa Explanation. NH 3 and NaOH. NaCl is a salt.
Notice that Cl- is common. Which solute combinations can make a buffer solution. CH 3 NH 2 and CH 3 NH 3 Cl.
CH 3 NH 2 and CH 3 NH 3 Cl. NH 3 and NaOH. Would hcl and nacl make a buffer.
HCHO 2 and NaCHO 2. By varying the amountquantity concentration of HCl andor NaCl the. You have an solution that is stoichiometric in ammonia.
CH 3 NH 2 and CH 3 NH 3 Cl. HCHO 2 is formic acid a weak acid while NaCHO 2 is the salt made from the anion of the weak acid the formate ion CHO 2. Answer 1 of 2.
HCl is a strong acid not a weak acid so the combination of these two solutes would not make a buffer solution. The combination of these two solutes would make a buffer solution. There is also neither.
Assume all are aqueous solutions. HCl and NaOH is not a butter solution. An example of a basic buffer is NH 4 OH and NH 4 Cl.
1 each 10 tablets. How exactly to make it. Dissolving one tablet in 500 ml of deionized water yields 150 mM NaCl 005 TWEEN 20 Detergent 50 mM Tris-HCl buffer pH 76 at 25C.
NH4OH HCl. No HCL and NaCl is not a buffer solution. Formic acid HCHO 2 is a weak acid while NaCHO 2 is a salt supplying formate ion CHO 2 the conjugate base of HCHO 2The combination of.
Formic acid HCHO 2 is a weak acid while NaCHO 2 is the salt made from the anion of the weak acidthe formate ion CHO 2 The combination of these two solutes would make. I asked my chem teacher and she said she didnt know sad huh. Assume all are aqueous solutions.
Consider the buffer systems equilibrium HClO rightleftharpoons ClO- H where K_a ClO-HHClO approx 3010-8 Moreover consider the ionization of water H_2O rightleftharpoons H OH- where K_w OH-H approx 1010-14 The preceding equations can be used to understand what happens when protons or hydroxide ions are added. HCl dissociates to H Cl- ions. I thought it would be a gas.
NaCl dissociates to Na Cl- ions. NaFaq HClaq -- HF NaClaq Apparently my answer was correct except that I didnt know what phase the HF would be. Im doing a protein extraction from muscle cells and I would like to know the function of these components of the lysis buffer.
NaCl is formed by the reaction of strong base NaOH and strong acid HCl. Immediately you add hydrochloric acid this protonates the ammonia and now you both NH_3 and NH_4 in some quantity. I just want to know why the buffer contains the NaCl.
Hydrochloric acid HCl is a strong acid not a weak acid so the combination of these two solutes would not make a buffer solution. Is HCl and NaOH a buffer. Supplied in convenient blister packs.
So I have to resort to a science forum for the answer. I use Tris-HCl ph75 50. Example PageIndex1 Which solute combinations can make a buffer solution.
HCl is a strong acid and NaCl is a salt of strong acid and strong base. If HCl and NaOH are mixed together the acid and the base will neutralise and form a neutral salt but it is not a buffer. What would the phase of the HF be.
NH_3aq HClaq rarr NH_4Claq Given ammonia and ammonium chloride.
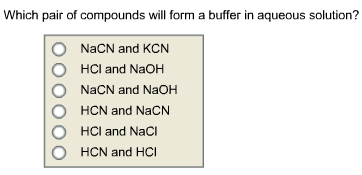
Which Pair Of Compounds Will Form A Buffer In Aqueous Solution Nacn And Kcn Hcl And Naoh Nacn Home Work Help Learn Cbse Forum
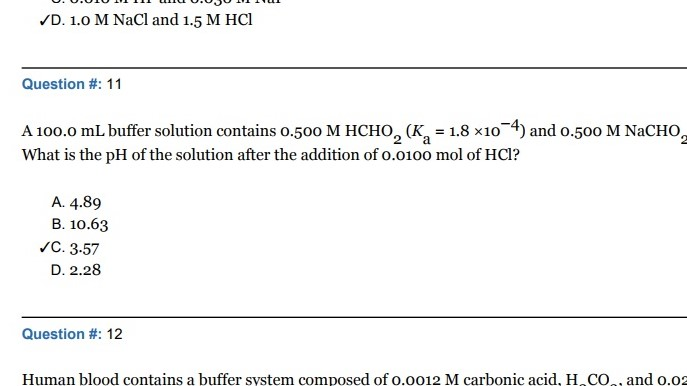
Solved D 1 O M Nacl And 1 5 M Hcl Question 11 A 100 0 Ml Chegg Com
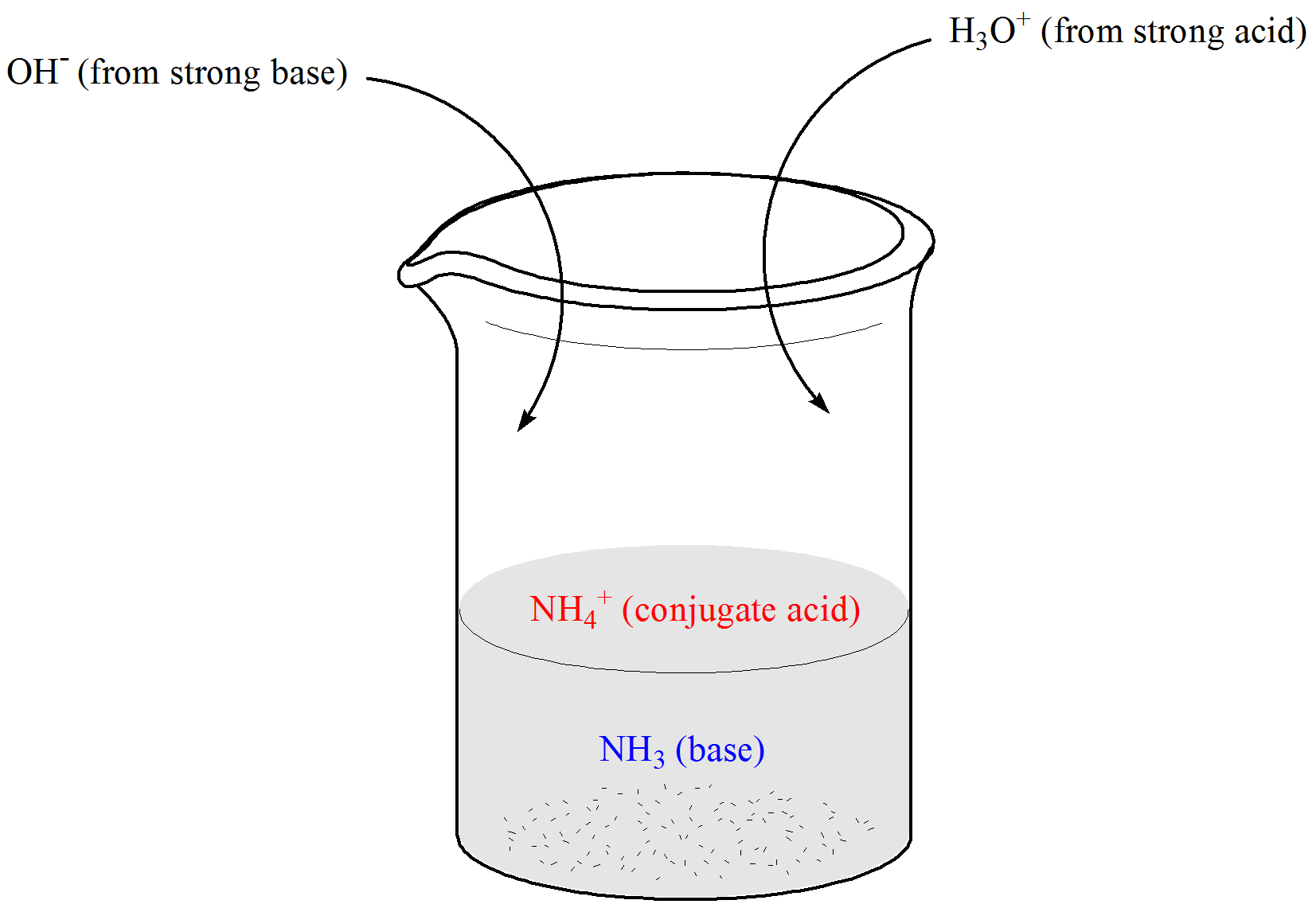
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy

Ph Calculation Of A Buffer Solution Made From A Weak Base And Its Conjugate Acid Salt Form Youtube
Why Would 2 Mol Ch3coona And 1 Mol Hcl Make A Buffer Solution Don T We Require A Weak Acid And Its Conjugate Base For A Buffer Quora
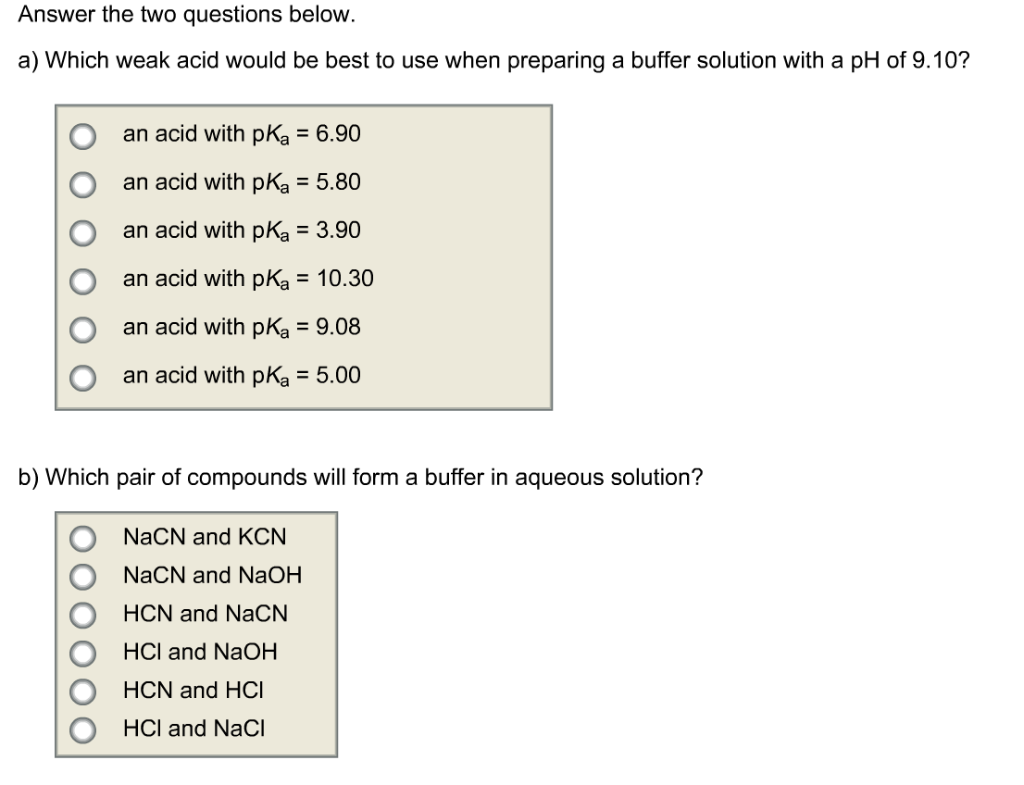
Solved Answer The Two Questions Below A Which Weak Acid Chegg Com

How To Calculate The Ph Of A Buffer Solution After Adding Acid Hcl Youtube

Calculate Ph Of Buffer After Adding Strong Base Youtube
What Is The Chemical Reaction When Acetic Acid Is Combined With Sodium Chloride Quora

20 10 11 Acid Base Buffers Applications Answers



Comments
Post a Comment